Many people in need of knee and ankle replacements have a decreased quality of life, with limited mobility and chronic pain. A successful joint replacement can be life-changing, with patients recovering from pain and regaining a sense of freedom once they’ve achieved full mobility.
Unfortunately, this is not always the case. Exactech had given many patients hope for a new beginning, only to leave them worse for the wear after their knee or ankle replacement surgery.
Why are Exactech products being recalled?
Exactech’s arthroplasty polyethylene inserts were made to alleviate joint issues, but many patients have had to undergo knee and ankle revision surgeries. It turned out that the product packaging for these inserts was faulty and exposed the inserts to high levels of oxygen. The oxidation weakened the insets prior to implantation, which caused them to wear and fracture easily. Exactech has issued a recall for these faulty devices.
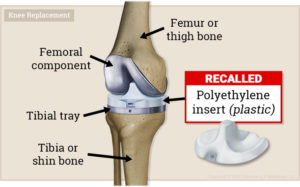
What injuries are associated with Exactech knee and ankle devices?
- Revision Surgery
- Bone Loss
- Loosening
- Swelling
- Instability
- Pain
What are some signs that you may be suffering from a faulty device?
- You hear a clicking or grinding sound while walking
- You feel unable to bear weight while walking
- Your joint pain has returned
- There is renewed swelling in the knee or ankle area post-surgery
Allen & Allen understands that when life-changing products become faulty, it can have large implications on your life. After overcoming chronic pain and immobility through surgery and rehabilitation, and taking into account the expense and time taken off from your career, it can be devastating to find yourself back at square one. Suffering a second time through another’s negligence is not a fair outcome.
If you have suffered from using an Exactech knee or ankle device, you may be entitled to compensation. Call the product liability attorneys at Allen & Allen today for a free consultation at 866.388.1307.


